MOUNTAIN VIEW, Calif. – May 8, 2024 — In a groundbreaking development, a study published in the Journal of Vascular Surgery reveals for the first time that coronary CTA (CCTA) with fractional flow reserve (FFRCT) care significantly reduces mortality by over 60 percent at five years in patients with PAD undergoing major vascular surgery, far surpassing the current standard of care.1
Atherosclerosis is a systemic disease that affects multiple vascular regions and is particularly severe in PAD patients, where up to 80 percent suffer from concurrent coronary artery disease (CAD), historically linked with a mortality rate exceeding 50 percent within five years.2-4 Heartflow’s non-invasive FFRCT technology has emerged as a leading frontline strategy for accurately diagnosing hemodynamically significant coronary lesions in patients suspected of having CAD.5 Revascularization to improve blood flow to the heart has been shown to reduce mortality in stable chest pain patients.6
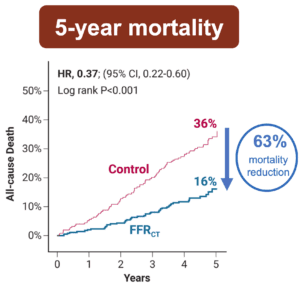
This novel study marks a significant milestone in the field, evaluating the effectiveness of FFRCT in detecting ischemia-producing coronary stenosis in patients with severe PAD. Among 522 surgical PAD patients with previously undiagnosed CAD, systematic FFRCT testing combined with targeted coronary revascularization resulted in a 63 percent reduction in all-cause mortality, an 89 percent decrease in cardiovascular death, and an 87 percent reduction in myocardial infarction during the five-year follow-up, compared to patients receiving standard cardiac evaluation.
“PAD affects over 230 million people globally, presenting a significantly underrecognized risk of CAD,” said Dr. Frank Arko, Chief of Vascular and Endovascular Surgery at Atrium Health Sanger Heart & Vascular Institute. “In an additional study looking at a similar patient population, we studied 170 high-risk PAD patients, where we utilized systematic FFRCT testing. Remarkably, 70 percent of these patients had hemodynamically significant CAD, which is a major risk factor for cardiac complications. This first-of-its-kind evidence firmly establishes the life-saving potential of FFRCT-guided diagnosis and treatment in improving long-term survival.”7
Heartflow leads the market in non-invasive CAD diagnosis and management. This publication showcases the company’s continued commitment to revolutionizing patient care with innovative and scientifically validated technologies.
Dr. Christopher Zarins, Senior Vice President of Medical Affairs at Heartflow, commented, “Despite advances in peripheral vascular disease treatment, the mortality rate following vascular surgery remains distressingly high. With our AI-powered approach, Heartflow addresses a critical and longstanding clinical challenge by noninvasively determining the presence of significant CAD and facilitating its effective treatment. Ultimately, the result is that patients with atherosclerotic disease can receive access to a more accurate diagnosis and treatment, dramatically reducing their likelihood of death.”
Heartflow is dedicated to defeating heart disease through partnering with physicians to generate robust, high quality clinical evidence. Heartflow has been adopted by over 1,000 institutions globally and continues to strengthen its commercial presence to make this cutting-edge solution more widely available to an increasingly diverse patient population worldwide.
The Heartflow FFRCT Analysis is part of a clinical pathway for evaluation and diagnosis of CAD that provides clear insight into a patient’s heart condition with a personalized visual model of the heart’s blood flow, helping physicians make more accurate diagnoses and treatment decisions.*
About Heartflow, Inc.
Heartflow is transforming precision coronary care with the only non-invasive integrated heart care solution across the CCTA pathway. As the pioneer of FFRCT, which is now supported by the ACC/AHA Chest Pain Guidelines, Heartflow continues to advance the diagnosis and management of CAD. Our suite of non-invasive technologies helps clinicians identify stenoses in the coronary arteries (Roadmap™Analysis), assess coronary blood flow (FFRCT Analysis), and characterize and quantify coronary atherosclerosis (Plaque Analysis). To date, more than 500 peer-reviewed publications have validated our approach and, more importantly, our technologies have helped clinicians diagnose and manage over 250,000 patients. For more information, visit www.Heartflow.com and connect on Twitter and LinkedIn.
Media Contact
Linly Ku
Heartflow
media@Heartflow.com
References
1 Krievins, Dainis K., et al. “Diagnosis and treatment of ischemia-producing coronary stenoses improves 5-year survival of patients undergoing major vascular surgery.” Journal of Vascular Surgery, Mar. 2024, https://doi.org/10.1016/j.jvs.2024.02.043.
2 Halliday, Alison, and Jeroen J. Bax. “The 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS).” European Journal of Vascular and Endovascular Surgery, vol. 55, no. 3, Mar. 2018, pp. 301–302, https://doi.org/10.1016/j.ejvs.2018.03.004.
3 Secemsky, Eric A., et al. “Longitudinal assessment of safety of FEMOROPOPLITEAL endovascular treatment with paclitaxel-coated devices among Medicare beneficiaries.” JAMA Internal Medicine, vol. 181, no. 8, 1 Aug. 2021, p. 1071, https://doi.org/10.1001/jamainternmed.2021.2738.
4 Bradbury, Andrew W, et al. “A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (basil-2): An open-label, randomised, Multicentre, phase 3 trial.” The Lancet, vol. 401, no. 10390, May 2023, pp. 1798–1809, https://doi.org/10.1016/s0140-6736(23)00462-2.
5 Gulati, Martha, et al. “2021 AHA/ACC/ASE/Chest/Saem/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.” Circulation, vol. 144, no. 22, 30 Nov. 2021, https://doi.org/10.1161/cir.0000000000001029.
6 Navarese EP, Lansky AJ, Kereiakes DJ, Kubica J, Gurbel PA, Gorog DA, et al. Cardiac mortality in patients randomised to elective coronary revascularization plus medical therapy or medical therapy alone: a systematic review and meta-analysis. Eur Heart J 2021;42:4638-4651.
7 Stanley, Gregory A., et al. “Utilization of coronary computed tomography angiography and computed tomography-derived fractional flow reserve in a critical limb-threatening ischemia cohort.” Journal of Vascular Surgery Cases, Innovations and Techniques, vol. 10, no. 2, Apr. 2024, p. 101272, https://doi.org/10.1016/j.jvscit.2023.101272.
*The Heartflow Analysis is an AI–based medical device software for the clinical quantitative and qualitative analysis of previously acquired Computed Tomography DICOM data for patients with suspected coronary artery disease. It provides anatomic data, plaque identification and characterization, as well as the calculations of FFRCT, a coronary physiological simulation, computed from simulated pressure, velocity and blood flow information obtained from a 3D computer model generated from static coronary CT images. The Heartflow Analysis is intended to support the risk assessment and functional evaluation of coronary artery disease.
The Heartflow Analysis is provided to support qualified clinicians to aid in the evaluation and risk assessment of coronary artery disease. The Heartflow Analysis is intended to be used by qualified clinicians in conjuncton with the patient’s clinical history, symptoms, and other diagnostic tests, as well as the clinician’s professional judgment.