
ISCHEMIA’s Ripple Effects and Where They Leave Cardiologists
This year’s American Heart Association (AHA) meeting did not disappoint as the highly anticipated results of the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial were presented. This NIH-funded global study focused on patients with myocardial ischemia documented by non-invasive functional testing, with 5,179 patients undergoing randomization (from 8,518 enrolled patients). It was conducted at 320 sites in 37 countries, and key findings were that:
- Through 3+ years of follow-up, coronary revascularization on top of optimal medical therapy (OMT) did not lower adverse event rates (MI, CV death, CV-related hospitalization, etc.)
- Patients with regular angina did however derive significant and lasting relief from revascularization as compared to OMT alone
Beyond these important results, what really caught my attention was that the ISCHEMIA investigators relied on coronary CT angiography (CCTA) to select patients and stratify risk. Incredible work was done to enroll patients with stable symptoms and moderate-to-severe myocardial ischemia in the trial, but the investigators rightly asked, “Can all of the enrolled patients safely be randomized?”
To answer that question, approximately 73% of patients underwent a blinded CCTA to determine who could and could not be randomized between ISCHEMIA’s revascularization and OMT-only arms. The impact is seen here:
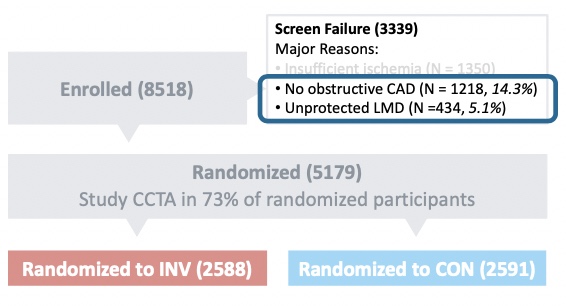
Based on the CCTA, the investigators excluded 434 patients who had unprotected left-main disease and another 1,218 patients who had no obstructive coronary disease despite having ischemia on non-invasive testing. This reinforces that CCTA informs clinicians in a manner that other diagnostic modalities simply cannot.
While we wait for published details of the ISCHEMIA trial findings to inform us more fully, many conversations have already turned toward the implications this trial will have on conventional non-invasive cardiac ischemia testing.
A question at the core of this discussion is “If functional stress testing doesn’t effectively guide treatment (i.e. help to select patients whose outcomes will improve with invasive treatment), why are we doing so many stress tests?”
Already, we know an important piece of the path forward will be to identify and effectively assess coronary anatomy and patient risk, as CCTA did in this trial. Such a path will be better for:
- Patients, as they get cardiac treatments they need and avoid what they don’t.
- Physicians, as they approach clinical decisions for challenging CV cases with better information.
- The health care system, as improved application of cardiac testing and treatments delivers efficiencies and savings that we need today.
The ISCHEMIA trial findings will play an important role as patient pathways move beyond stress tests focused on myocardial function and integrate a focus on lesion-specific anatomy and function through a CCTA+FFRCT guided pathway.
— A perspective from HeartFlow Chief Medical Officer, Campbell Rogers, MD
Bio | LinkedIn





